LSI spread function returns a water stability index (Langelier Saturation Index) given a pH, TDS, Calcium, and Water Temperature (Deg C). The Langelier Saturation Index (LSI) is a means of evaluating water quality data to determine if the water has a tendency to form a chemical scale. In order to use this index, the following laboratory analysis are needed: pH, conductivity, total dissolved solids, alkalinity, and total hardness. In manipulating the data, the actual pH of the water is compared to the theoretical pH (pHs) based on the chemical analysis. LSI = pH - pHs
SYNTAX:
LSI(pH_actual, TDS,DegC, Calcium, Alkalinity )
where :
- pH_actual: pH measurement of the water
- TDS: The Total Dissolved Solids (mg/L)
- Calcium: Calcium (mg/L)
- Alkalinity: Alkalinity (mg/L)
- DegC: Water temperature (deg C)
NOTES:
WIMS calculates LSI based on the following equation:
LSI = pH - pHs
The Saturation Index is typically either negative or positive and rarely 0. A Saturation Index of zero indicates that the water is “balanced” and is less likely to cause scale formation. A negative LSI suggest that the water is would be undersaturated with respect to carbonate equilibrium and the water may be more likely to have a greater corrosive potential..
Corrosive water can react with the household plumbing and metal fixtures resulting in the deterioration of the pipes and increased metal content of the water. This reaction could result in aesthetic problems, such as bitter water and stains around basins/sinks, and in many cases elevated levels of toxic metals. A positive SI suggests that water may be scale forming. The scale, typically a carbonate residue, could clog or reduce the flow in pipes, cause buildup on hot water heaters, impart an alkali taste to the water, reduce the efficiency of the water heaters, and cause other aesthetic problems.
· pH is the measured water pH
· pHs is the pH at saturation in calcite or calcium carbonate and is defined as:
pHs = (9.3 + A + B) - (C + D)
Where:
A = (Log 10 [TDS] - 1) / 10
B = -13.12 x Log10 (oC + 273) + 34.55
C = Log10 [Ca2+ as CaCO3] - 0.4
D = Log10 [alkalinity as CaCO3]
See also: CCPP
EXAMPLE:
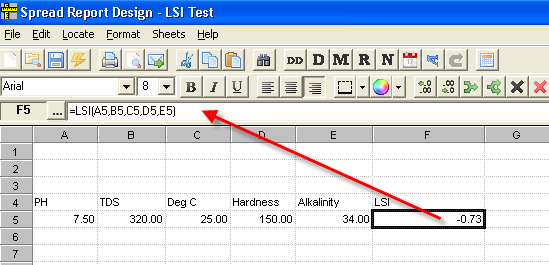
LSI(A5,B5,C5,D5,E5) where
- A5 is a cell with pH
- B5 is a cell with Total Dissolved Solids (mg/L)
- C5 is a cell with Water Temp in Deg C
- D5 is a cell with Calcium Hardness as CaCO4 mg/L
- E5 is a cell with Alkalinity (mg/L)
Example:
For further information about the Langelier Saturation Index:
http://www.corrosion-doctors.org/Cooling-Water-Towers/Index-Langelier.htm